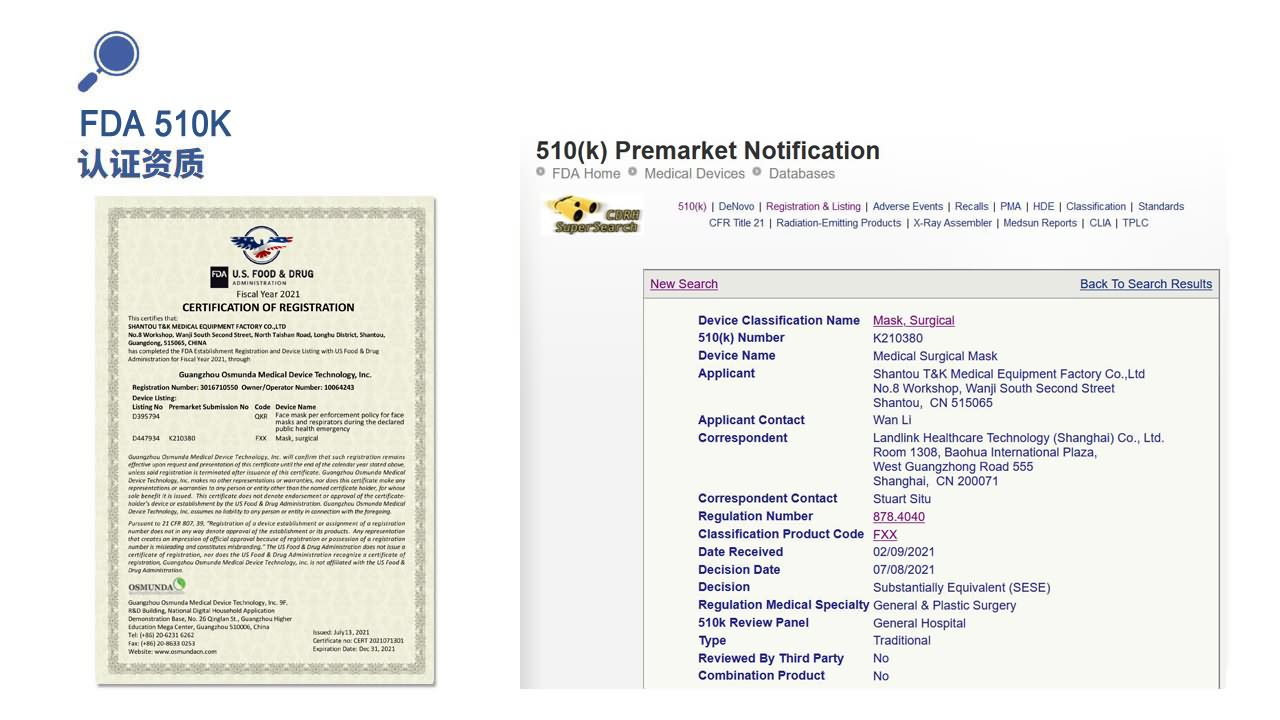
The company is a subsidiary of Guangdong T&K Pharmaceutical Co., Ltd. (Stock Code: 301263), specializing in the research, development and production of medical devices for 20 years, certified by ISO 9001:2015, ISO 13485:2016, CE, FDA, TGA, WHO-GMP, EN 14683:2019+AC:2019, etc... The products cover 28 provincial regions, thousands of hospitals, and about 90,000 pharmacies, the production of "T&K" brand medical supplies is well received by the market at home and abroad.
Our main products include medical masks, medical surgical masks (ear-loop and tie-on type), kids medical surgical masks, KN95 / FFP2 protective masks, KF94 fish shape protective masks, disinfecting wet wipes, disposable medical alcohol pads, medical cotton swabs, and so on, now has class 100K clean workshop, aseptic testing room, positive control room and microbial limit room and a series of professional testing equipment, to ensure that each piece of mask product conforms to the strict quality standard, more than 900 million masks have been supplied worldwide during the outbreak!